For supervised testing
CareStart™ COVID-19
rapid antigen point-of-care test.
Reliable, rapid lateral flow immunochromatographic assay for the qualitative detection of nucleocapsid antigen from SARS-CoV-2 present in nasal samples.
Results in 10 minutes
The CareStart COVID-19 Antigen (RAT) provides results in just 10 minutes, making it highly suitable for quick action at the point-of-care. Anterior (shallow) nasal sampling collects from the front area of the nose and is therefore less invasive, reduces patient discomfort and simplifies the overall testing procedure.
Anterior nasal swab samples may be particularly useful for children and elderly people, especially when used for frequent testing. This simplified collection method also allows for the option for patients to self-collect under supervision, offering reduced physical contact and exposure and further protection for healthcare practitioners.
Negative test results do not exclude infection with COVID-19 (so face masks, social distancing and good hygiene practice must be maintained). Always follow current government health messaging regarding polymerase chain reaction (PCR) testing requirements.
Available for order and immediate delivery
Bulk CareStart™ point of care RAT tests are available for order. Submit and online order request, or talk to one of our friendly staff.
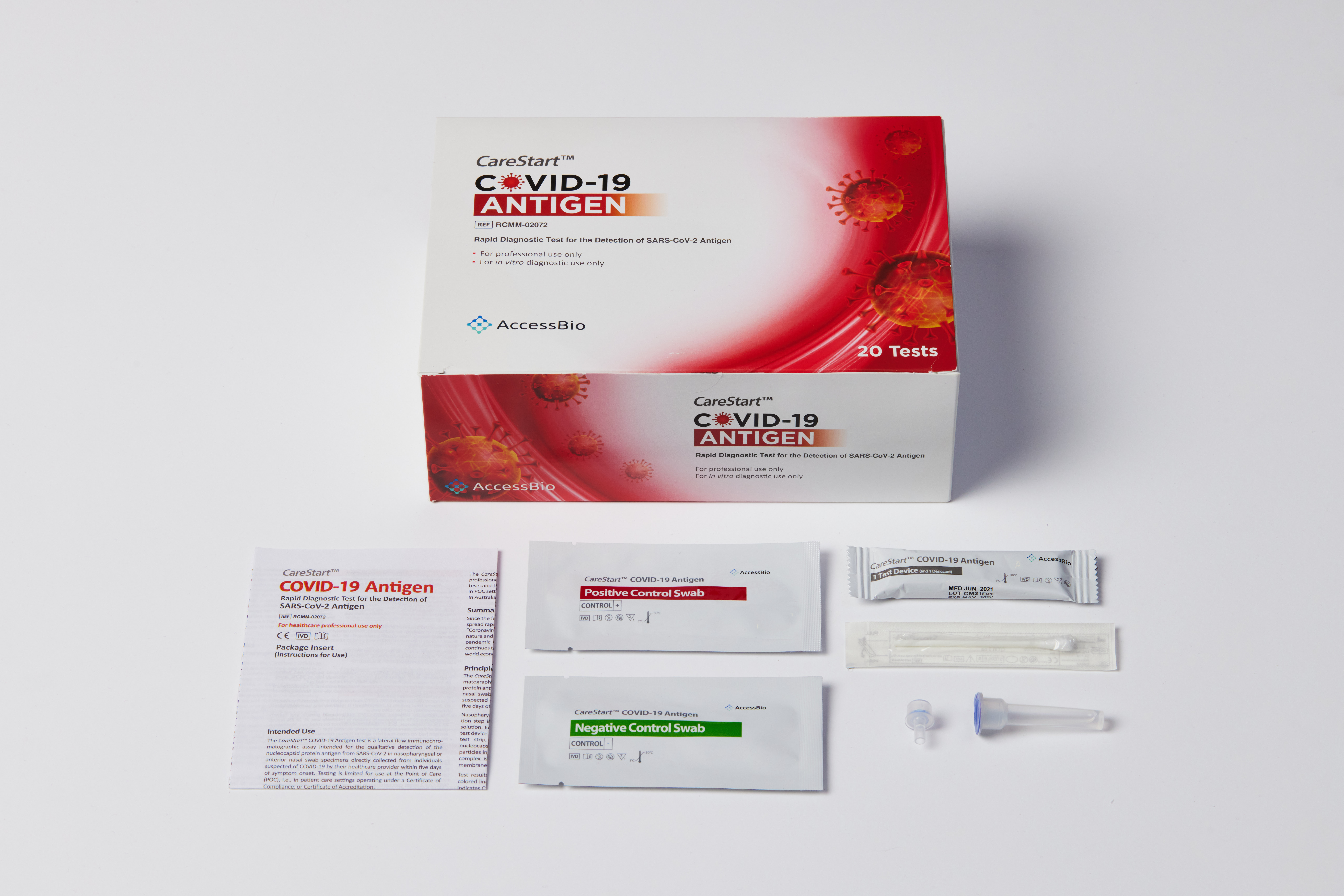
Test components
All components are included in the kit (20 tests per kit):
- 20 x test device (individually wrapped in a foil pouch)
- 20 x pre-prepared extraction vials with buffer solution and 20 x vial caps
- 20 x sterile anterior nasal swab
- Instructions for use and quick reference guide
- Positive and negative controls
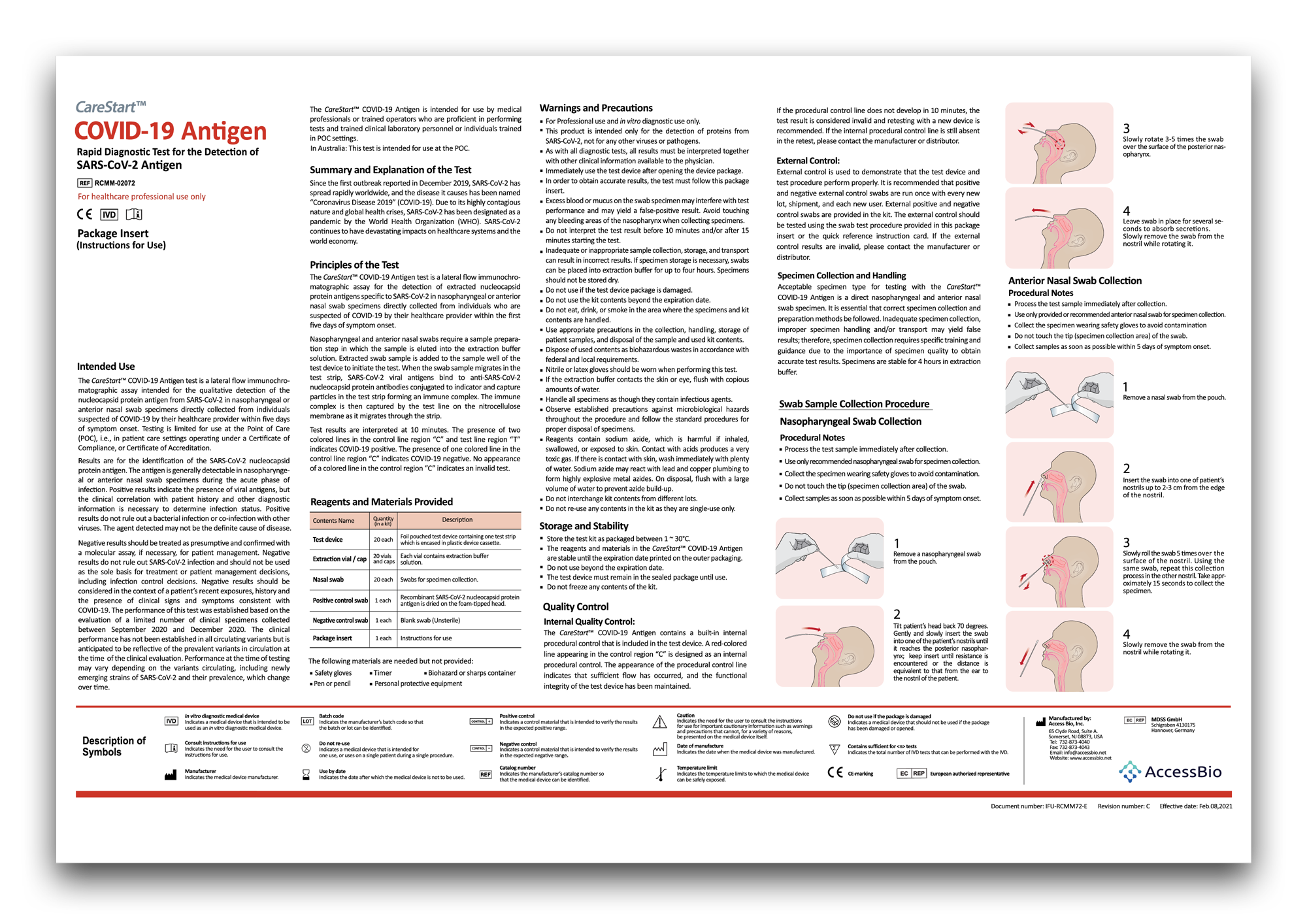
Instructions & training
Always follow the instructions for use when using the CareStart™ COVID-19 Antigen Tests.
Regulatory information
Registration of CareStart™ COVID-19 Antigen
Entered in the Australian Register of Therapeutic Goods, 342512
The TGA requires that rapid antigen tests are currently supplied under specific conditions. These include for use by trained health practitioners, and trained staff under their supervision, to ensure a suitable health practitioner is available to provide immediate clinical advice and treatment if required. See below to read the full conditions of supply.
The device cannot be supplied for self-testing (home-use).
Conditions of supply
‘The TGA requires that rapid antigen tests are currently supplied under specific conditions. These include for use by trained health practitioners, and trained staff under their supervision, to ensure a suitable health practitioner is available to provide immediate clinical advice and treatment if required.
Testing can be performed by persons who are not a health practitioner however testing needs to be performed under the overall supervision of a health practitioner, medical practitioner or paramedic, and the person performing the test has been trained in the correct use and interpretation of the tests. Where it is not possible for a health practitioner to be physically present, the supervision does not need to be direct.
Everyone who will perform the test needs to be trained in the correct use of the device (including specimen collection) and interpretation of results. This training needs to be undertaken prior to commencement of any testing.
Use of the test by untrained persons and testing performed outside the supervision of a health practitioner would mean that the person or organisation could be liable if something goes wrong with the performance or interpretation of the test.
This device cannot currently be supplied for self-testing (home-use).’
TGA FAQ
Q&As - Conditions of supply for rapid antigen point of care COVID-19 tests